Bears Make the Best Science Buddies
Author: Carmen Oliver and Jean Claude (Illustrator)
Publisher: North Mankato, Minnesota: Capstone Editions, a Capstone imprint, 2020

|
For more resources on science for preschoolers, please click here!
|

Do you know what happens when you mix baking soda and vinegar? Let’s learn about chemical reactions and the gas they produce when they are mixed. Observe how a balloon can be inflated through this simple experiment.
Chemical reaction: A process that involves the arrangement of molecules or structure of a substance, as opposed to a change in physical form (as from a solid to a gas).
Chemical Reactions Facts. Cool Kid Facts. (2022). Retrieved 28 March 2022, from https://www.coolkidfacts.com/

Watch these videos to find out how a balloon can be inflated through this simple experiment.
Blow Up a Balloon with Science (4min 24 secs)
Can you believe that you can blow up a balloon without actually blowing your own air into it? Follow along with this super neat experiment and find out how!
Blow Up a Balloon with No Hands Experiment (2min 50 secs)
Learn how you can blow up a balloon with a simple science experiment!
Have you been to a party with balloons? Can you blow a balloon without using your mouth or an air pump? Sure you can!
In this simple science experiment, you will discover how you can inflate a balloon without blowing it using only a few everyday household items that you probably already have at home. You just need a chemical reaction to produce carbon dioxide gas and get ready to watch a fizzy reaction!
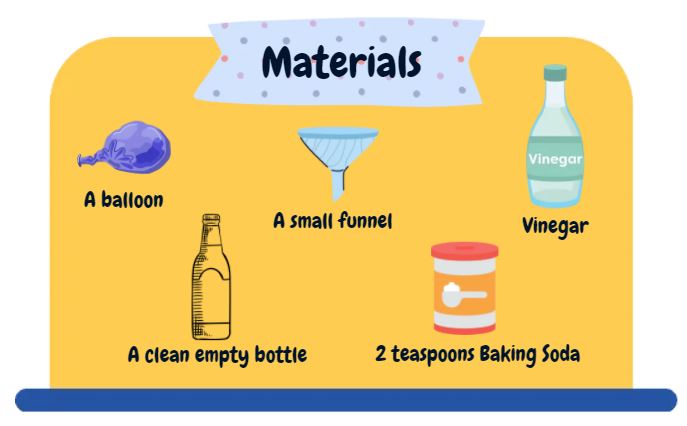
Ask your child to predict what will happen when you combine baking soda and vinegar in a bottle. Ask your child to predict what will happen if you combine the same ingredients, but cover the top of the bottle with a balloon.
1. Using the funnel, add 1-2 teaspoons of baking soda into the balloon.
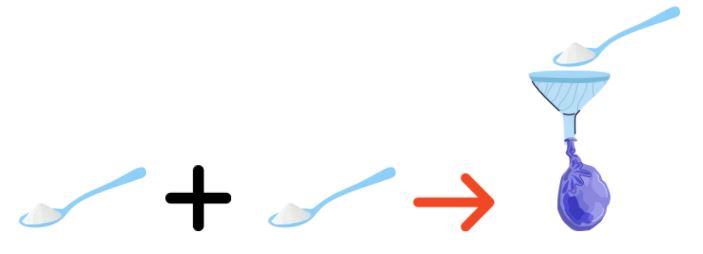
2. Pour the vinegar into the bottle until it is half-filled.
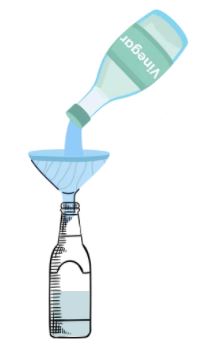
3. Carefully fit the balloon lip over the bottle’s nozzle, while holding onto the balloon.
(Be careful not to drop the baking soda into the vinegar yet).
4. Once the balloon is fitted snugly on the nozzle, and you are ready, hold up the balloon and allow the baking soda to fall into the vinegar.
5. Observe the chemical reaction and effect on the balloon.


Before starting the experiment, you might want to stretch the balloon tip to make it more elastic and easier to inflate.
Record and share your observation and findings using this template.
1. What did you hear?
2. What did you see?
3. How did the balloon inflate?
4. How does the chemical reaction works?
Making carbon dioxide gas (CO₂) from vinegar and baking soda: This experiment explores how a gas takes the shape of the container it fills, in this case, a balloon. If you mix vinegar and baking soda, a chemical reaction will occur, where lots of bubbles of carbon dioxide gas form.
The chemical reaction of sodium bicarbonate (baking soda) and ethanoic acid (vinegar) produces carbon dioxide gas. The carbon dioxide gas can originally be seen as bubbles in the solution, but will quickly be released from the solution. The carbon dioxide gas will flow within the spaces in the bottle, and move into the deflated balloon, filling the balloon and thus inflating it.
You can do this reaction at home but make sure you don’t get any of the chemicals in your eyes. Ethanoic acid is the chemical name for vinegar. Sodium bicarbonate is also known as baking soda.
Source: Primary Connections: Linking science with literacy. Primaryconnections.org.au. (2022). Retrieved 28 March 2022, from https://www.primaryconnections.org.au/themes/custom/connections/assets/SBR/data/Chem/sub/baking/baking.html
Reusing the vinegar will not work. You will have to rinse the bottle each time and pour fresh vinegar into the bottle before starting again.
The reaction from the baking soda and vinegar is rapid. Ensure your child is ready to see the effect before combining the two items.
You may reuse the balloons, but eventually the neck of the balloons will wear out and rip from use.
Do the experiment multiple times, while changing the different variables.
1. What if more baking soda is used this time, with the same amount of vinegar?
2. What if more vinegar is used next, with the same amount of baking soda? Which chemical reaction inflate the biggest balloon?
Record and share your observation and findings using the template provide: https://go.gov.sg/observationtable1
https://go.gov.sg/observationtable2

You may prompt the following questions to your child during or after the experiment:
Did you see bubbles floating on top of the baking soda and vinegar mixture? Why? This experiment illustrates how states of matter can change; A chemical reaction takes place when baking soda is mixed with vinegar. Carbon dioxide or CO2 is released and inflates the balloon.
Do you know what gas you blow into the balloon to inflate it? How is this gas produced? When you blow up a balloon, you are exhaling carbon dioxide into the balloon. We breathe in oxygen and breathe out carbon dioxide. It is the a biproduct of our respiratory system.
What else can you fill up with a gas?
Source: Carbon Dioxide Facts For Kids | Among First Discovered Gases. Facts For Kids. (2022). Retrieved 29 March 2022, from https://factsforkids.net/carbon-dioxide-facts-for-kids/

Bears Make the Best Science Buddies
Author: Carmen Oliver and Jean Claude (Illustrator)
Publisher: North Mankato, Minnesota: Capstone Editions, a Capstone imprint, 2020

Where is My Balloon?
Author: Ariel Bernstein and Scott Magoon (Illustrator)
Publisher: New York: Simon & Schuster Books for Young Readers, 2019

Inflating a Balloon
Author: Brooke Rowe and Jeff Bane (Illustrator)
Publisher: Ann Arbor, Michigan: Cherry Lake Publishing, 2018

Where is the Gas?
Author: Marla Conn
Publisher: Vero Beach, FL: Rourke Educational Media, a division of Carson Dellosa Education, 2021

States of Matter
Author: Samantha Bell and Jeff Bane (Illustrator)
Publisher: Ann Arbor, MI: Cherry Lake Publishing, 2018

Matter
Author: Abbie Dunne
Publisher: North Mankato, Minnesota: Capstone Press, 2017
All book covers are copyright of the respective publishing companies.
Look for these wonderful books and more on the NLB Catalogue!
Can’t get enough? Don’t put aside your everyday household items away, check out these other related exciting science experiments that you can do, and all thanks to science! Have fun!
Suitable for 4 to 6 years old
Whether it is bath time or dishwashing time, your child would be exposed to liquid soap and soap foam. Why not take these moments as opportunities to spark their curiosity about the densities of everyday objects? Read on to introduce your child to the concept of density, allow them to experiment with household liquid materials, and create ‘fireworks’ in a bottle together!

Density is a measurement that compares the amount of matter an object as to its volume.
(Density Facts for Kids. (2021). Density Facts for Kids. Retrieved December 28, 2021, from https://kids.kiddle.co/Density)
Use these printables to further explain density to your child!
|
Try introducing these words to your child! Here are some example sentences to incorporate: |
|
|---|---|
|
Liquid |
Liquids do not have a definite shape, so they take up the shape of the container they are poured into. Water and oil are liquids. |
|
Solid |
Solids have a definite shape, so they do not change shapes. For instance, this bowl that we are holding is a solid. |
|
Float |
Let’s put this pencil into water. Do you see that it floats on water? |
|
Sink |
Try putting this coin into water. It sinks all the way to the bottom! |
|
Molecules |
Molecules are very small substances that make up an object. You cannot see molecules with your eyes! You would need a microscope. |
|
Density |
The density of an object depends on how many molecules make up the object. |
Constant repetition and exposure are key in not just improving vocabulary but also in giving children the confidence to pick up unfamiliar words.
What is Density? | Science for Kids (1 min 57 secs)
In this 2-minute video, learn more about what density is and how it affects objects in water - no matter their weight or size!
How to Make Salt or Sugar Water Density Rainbow Tower | Simple Kids Science (1 min 49 secs)
Take it a step further and try this saltwater density rainbow tower with your child! How does salt affect the density of water?
Materials required:

Steps
1. Fill the bottle with water.
2. In the small bowl, add 4 tablespoons of vegetable oil and about 12 drops of food colouring. Stir lightly with the spoon and break up the drops of food colouring.
3. Pour the bowl of oil and food colouring into the bottle of water.
4. Observe what happens and allow your child to record their observations by ticking and circling in the table below. Experiment with other objects and liquids too!
5. (Optional) Print out the Observations Table to go through and record the observations with your child.


By having your child predict and record their observations, you are helping them develop their process-thinking as they experiment, analyse, and draw conclusions.

Rosa's Big Boat Experiment
Author: Jessica Spanyol
Publisher: Swindon: Child's Play (International), 2020

Will It Float or Sink?
Author: Lisa J. Amstutz
Publisher: North Mankato, Minnesota: Pebble, a Capstone imprint, 2022

Boats Will Float
Author: Andria Warmflash Rosenbaum & Brett Curzon (Illustrator)
Publisher: Ann Arbor, MI: Sleeping Bear Press, 2020

Why Do Ice Cubes Float?
Author: Benjamin Proudfit
Publisher: New York : Gareth Stevens Publishing, 2016
The copyright to all book covers belongs to the relevant publishers or illustrators.
Look out for these wonderful books and more on the NLB Catalogue!
Have a fun day at the swimming pool and inflate a float together with your child! Point out how the float stays afloat after inflation. Ask your child what they think is happening.

Have you ever wondered why apple slices turn brown when they are exposed to air for a period of time? What can you do about it? Let’s find out with a simple experiment that you can do at home.

There is oxygen all around us in the air we breathe. Iron is permeable, which means liquid or air can pass through it. So when it is exposed to water or air, a chemical reaction occurs and it changes to iron oxide (iron + oxygen) and begins to break down.
(Oxidation Facts for Kids. (2022). Oxidation Facts for Kids. Retrieved February 22, 2022, from https://kids.kiddle.co/Oxidation)
#GSCAtHome | Apple Oxidation (4 min 10 secs)
In this 4-minute video, learn more about oxidation, why is it important and what it does to cut apples.
Science Buddies | Write Secret Messages With Invisible Ink! (2 min 28 secs)
Take it a step further and try writing secret messages to your best friend or even to your family!
Objective: To explore the chemical reaction behind apple browning. We will also test to see whether or not salt water, lemon water, and other liquids that can be found in the kitchen keep apples from browning.
Materials required:
.jpg)
Print out the Observation Table to go through and record the observations with your child.
Steps
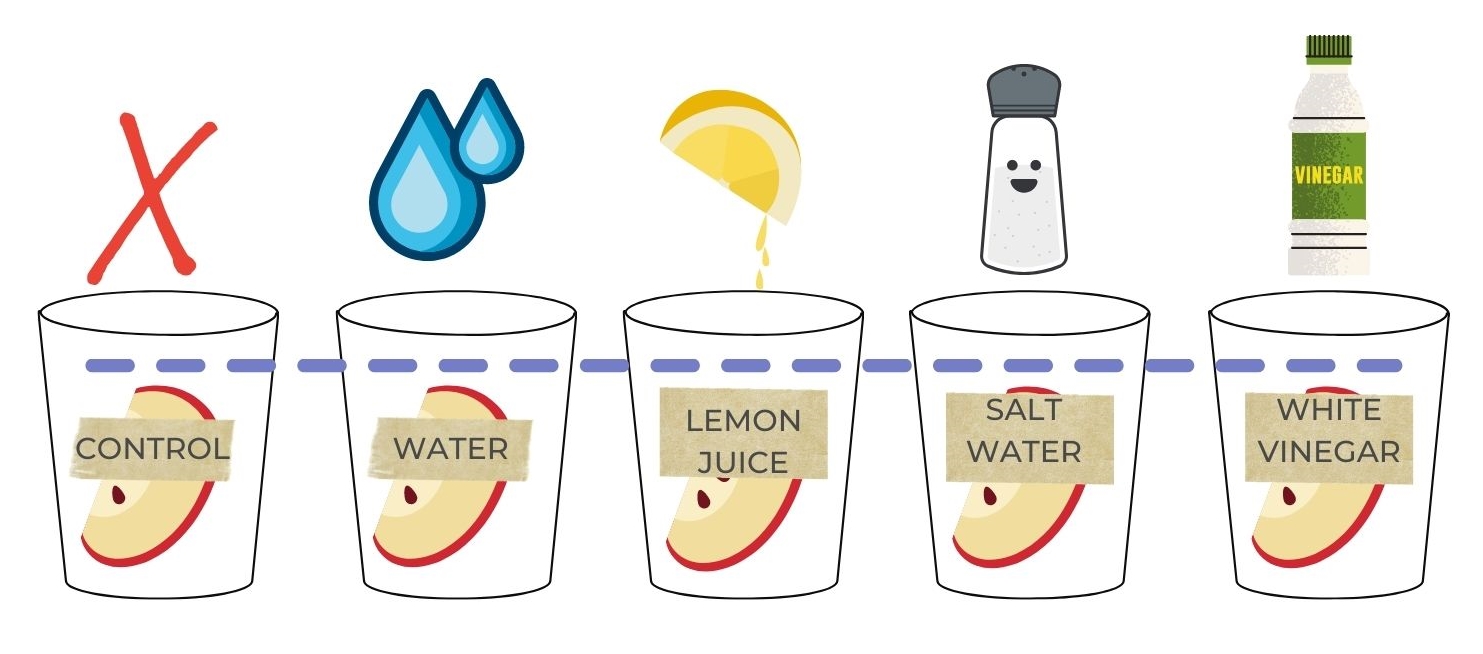
1. Label the cups/bowls and plate.
Control
Water
Lemon juice
Vinegar
Salt water

2. Cut the apple into 5 wedges
3. Place a wedge in each cup/bowl. Pour the liquids in the respective cup/bowl. Let the apples soak for 5 mins.
4. After that, take out the apples and placed them onto the plate that has already been labelled.
.jpg)
5. Observe what happens and allow your child to record their observations by colouring the template provided. Record the findings at 5 mins and 15 mins.
You may also prompt the following questions to your child:
How long did the apples take to turn brown?
Which apple turned brown first?
How dark did the apples get? Did the apples turn an equal shade of brown?
Go ahead and eat the apple slices! Do the apple slices taste different? Compare the taste of the apple slice coated in lemon juice with the plain apple slice? Which one tastes better
Do you think the lemon juice slowed or quickened the browning process?
By having your child predict and record their observations, you are helping them develop their process-thinking as they experiment, analyse, and draw conclusions.

Libby Loves Science: Mix and Measure
Author: Kimberly Derting and Shelli R. Johannes
Publisher: New York, NY: Greenwillow Books, an imprint of HarperCollinsPublishers, 2021

Look: I'm a Scientist
Author: Helene Hilton
Publisher: London : Dorling Kindersley Limited, 2017.
The copyright to all book covers belongs to the relevant publishers or illustrators.
Look out for these wonderful books and more at the NLB Catalogue!